
Biofidus News & Updates
Stay tuned for the latest developments and insights
FDA – Waives Clinical Efficacy Studies
A New Era for Biosimilars: Powered by Analytics
In a historic regulatory shift, the U.S. FDA has reportedly accepted the first application for a biosimilar monoclonal antibody without requiring a comparative clinical efficacy study.
This landmark decision signals the beginning of a new era in biosimilar development, where scientific rigor and affordability converge. It also aligns the FDA with the European Medicines Agency (EMA) and the UK’s MHRA, which have already embraced streamlined, evidence-based pathways for biosimilar approval.
At Biofidus, we see this as a transformative opportunity for the biopharmaceutical industry. With clinical efficacy studies no longer a default requirement, the burden of proof lies largely with analytical comparability, the domain where Biofidus excels. Our advanced analytical platforms and deep expertise in structural and functional characterization of protein-based therapeutics position us as your ideal partner in biosimilar development.
There is scientific consensus that a robust data package, consisting of analytical comparability studies and an appropriately designed pharmacokinetic (PK) study, will in most cases provide sufficient evidence of biosimilarity. Comparative efficacy trials have repeatedly failed to provide meaningful additional information, while analytical methods have proven to be more sensitive and predictive. A recent case study on ustekinumab biosimilars confirmed that single-dose pharmacokinetic studies and analytical data alone are sufficient to draw conclusions about comparable immunogenicity.
The above underscores the central role of analytical comparability in modern biosimilar development and empowers developers to focus resources on robust analytical packages, accelerating timelines and reducing costs by up to 90%
Biofidus supports this evolution with tailored comparability studies that meet the highest regulatory standards. From glycan profiling and charge variant analysis to potency assays as well as surface plasmon resonance, our services are designed to uncover the critical quality attributes that define biosimilarity. We help you build the totality of evidence regulators now prioritize.
As the biosimilar landscape opens to mid-sized and generic companies, Biofidus stands ready to guide your journey. Let’s redefine biologics together—through science, speed, and smarter development.
Interested in our biosimilar services?
https://www.biofidus.de/studies/biosimilar-studies/
References and additional resources:
“Professor Sarfaraz K. Niazi Secures First-Ever FDA Acceptance to Waive Clinical Efficacy Studies for Monoclonal Antibody Biosimilars” PR Newswire, https://www.prnewswire.com/news-releases/professor-sarfaraz-k-niazi-secures-first-ever-fda-acceptance-to-waive-clinical-efficacy-studies-for-monoclonal-antibody-biosimilars-302542781.html. Accessed 5 Sept. 2025
Guillen E, Barry S, Jost N, Ekman N, Knippel V, Kuhlmann-Gottke J, Maier J, Weise M, Laslop A, Anour R, van Zandbergen G, Kirsch-Stefan N. The Tailored Biosimilar Approach: Expectations and Requirements. Drugs. 2025 May;85(5):601-608. doi: 10.1007/s40265-025-02168-y. Epub 2025 Apr 1. PMID: 40169518; PMCID: PMC12031863.
Niazi S. Scientific Rationale for Waiving Clinical Efficacy Testing of Biosimilars. Drug Des Devel Ther. 2022 Aug 24;16:2803-2815. doi: 10.2147/DDDT.S378813. PMID: 36043044; PMCID: PMC9420434.
Schiestl M, Roy N, Trieb M, Park JP, Guillen E, Woollett G, Wolff-Holz E. Analytical Data and Single-Dose PK are Sufficient to Conclude Comparable Immunogenicity for Biosimilars: An Ustekinumab Case Study. BioDrugs. 2025 Sep;39(5):769-776. doi: 10.1007/s40259-025-00733-1. Epub 2025 Jul 17. PMID: 40676496; PMCID: PMC12354544.3
A holistic analysis platform for assessing protein modification and functional activity
Linking Protein Modifications to Functional Impact.
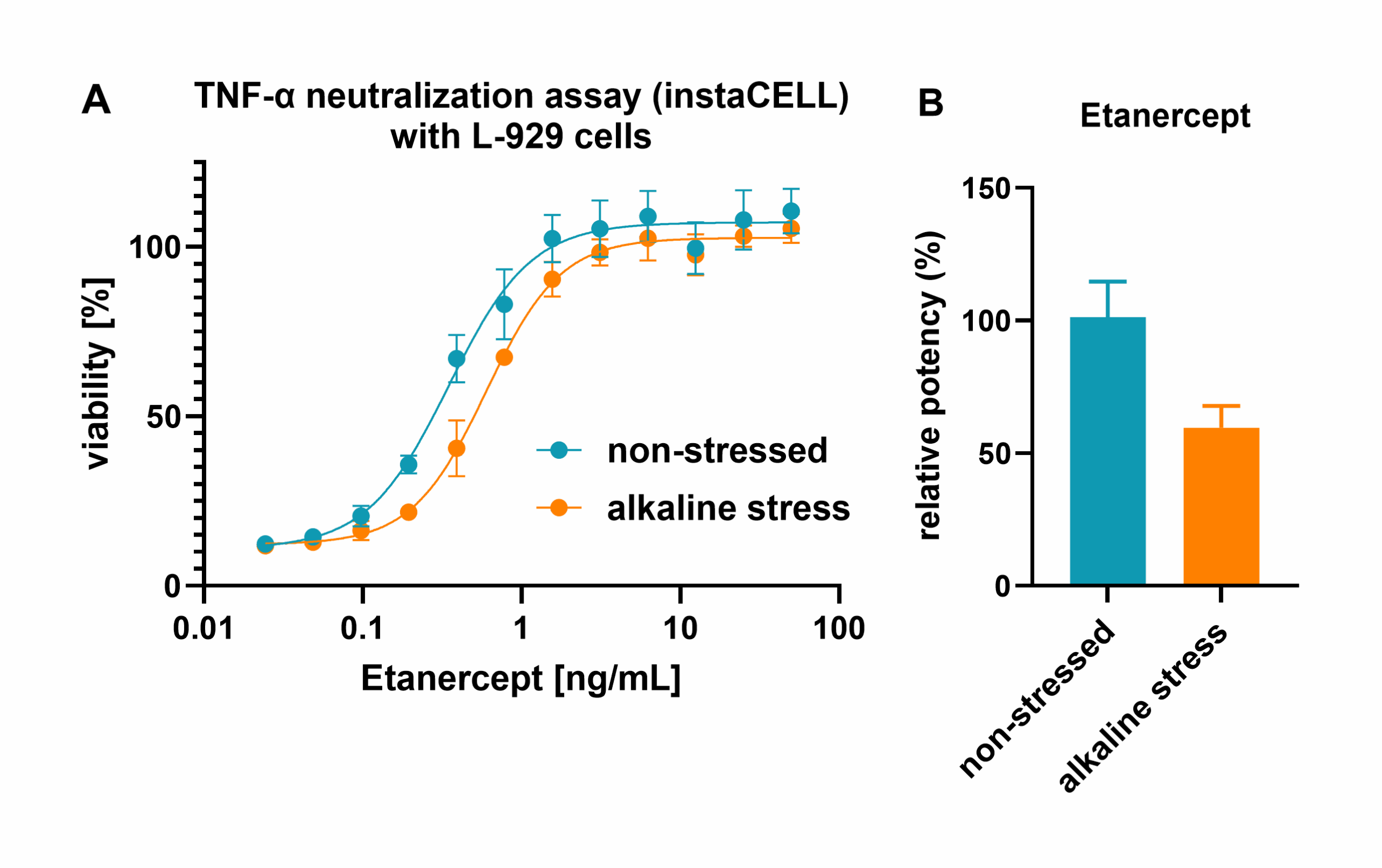
Protein modifications are critical for the efficacy, safety, and stability of therapeutic antibodies. Here, we introduce a comprehensive analysis platform tailored to assess post-translational modifications of therapeutic proteins hand in hand with their functional activity. In the present approach, we successfully applied peptide mapping with LC-ESI-MS to determine deamidation and oxidation of alkaline-stressed Etanercept. On functional level, the instaCELL TNF-α neutralization assay served as a state-of-the-art cell-based assay to assess the potency of Etanercept. With our holistic analysis platform, we observed increased deamidation accompanied by a loss of functional activity of Etanercept upon alkaline stress, making our approach a blueprint for future characterization studies of therapeutic antibodies.
Download whitepaper: CBA_Etanercept
Midas Pharma and Biofidus join forces for integrated Biotech Solutions
Biofidus AG is now a member of the Midas group.
With the acquisition of Biofidus, a leading provider of structural and functional characterization of biopharmaceuticals, Midas Pharma has taken a major step forward in expanding its portfolio of biopharmaceutical services.
With this merger, the companies expand their portfolios of service solutions, offering seamless and modular support for the entire pharmaceutical value chain – from discovery to commercialization.
Joining forces provides new opportunities for future growth, innovation, and shared success. As key part of their mutual growth strategy, the companies are implementing a GMP environment at Biofidus to further expand their analytical capabilities.

About Midas Pharma
Midas Pharma is a pharmaceutical company based in Ingelheim, Germany, that offers products, services, and expertise along the entire pharmaceutical value chain from Starting Materials and Active Pharmaceutical Ingredients to market ready Finished Products and Devices.
For over three decades the family-owned company has successfully contributed to the Pharma sector and has step by step expanded its competencies. With more than 300 employees and 12 locations in all major pharmaceutical markets worldwide, Midas Pharma has excellent local know-how, local contacts, and well-established global networks in different pharmaceutical sectors (biological products, small molecules and medical devices).
Thanks to many years of experience and extensive expertise, Midas Pharma has a proven track record of successfully supporting its customers in coordinating even complex pharmaceutical projects and creating significant added value.
About Biofidus
Biofidus AG, headquartered in Bielefeld, Germany, is a leading provider of structural and functional characterization of biopharmaceuticals founded in 2015 by Dr. Benjamin Müller and a team of experienced scientists.
Driven by a deep understanding of market trends, including the growing preference for outsourced analytics, Biofidus was established to deliver comprehensive analytical services to the pharmaceutical industry. With a team of 30 interdisciplinary experts, Biofidus has positioned itself as a reliable and knowledgeable partner, guiding businesses from start-ups to big pharma.
With a wide range of advanced analytical methods for structural and functional characterization of proteins and gene therapeutics, Biofidus strives to fast-track the development of innovative biologicals and biosimilars from early discovery to preclinical stage.
For further information on both companies, see www.midas-pharma.com and www.biofidus.de