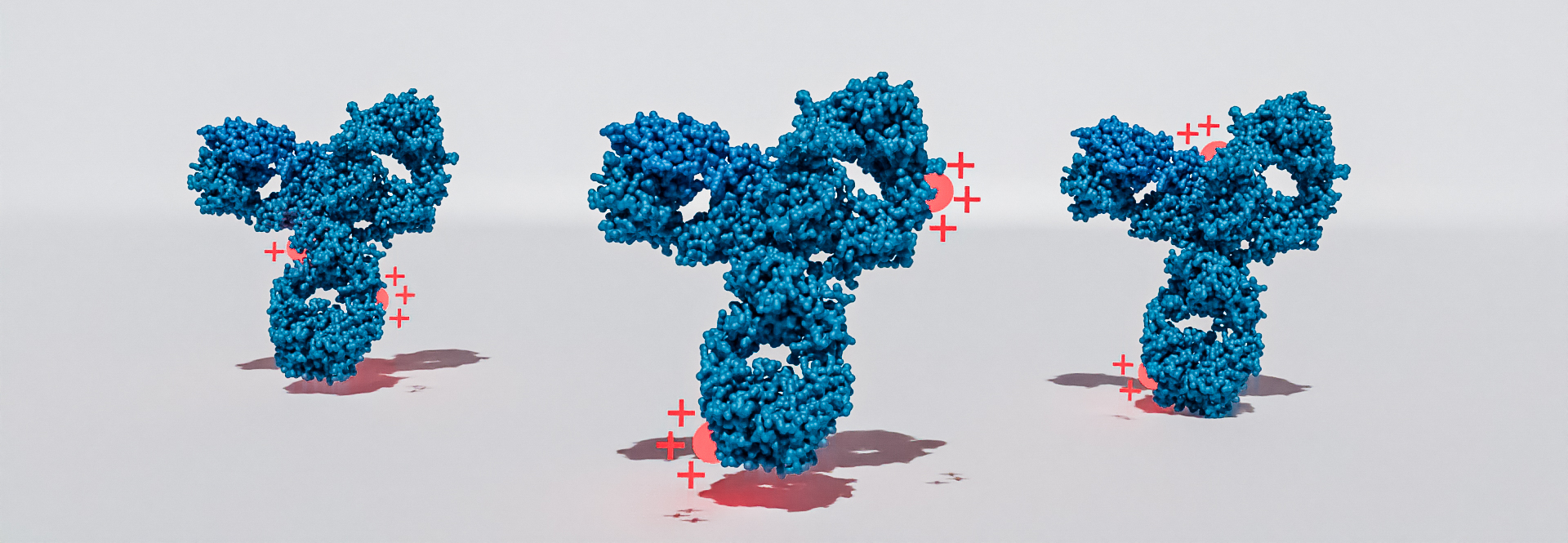
Charge heterogeneity
The monitoring of charge heterogeneities of protein-based drugs is an integral part of any quality and product lifecycle management plan. Biologics are susceptible to various enzymatic and chemical post-translational modifications (PTMs) during their production process. These PTMs can significantly change the charge pattern and consequently lead to an alteration of the physical and chemical properties of a protein, such a solubility, stability, and binding affinity. To guarantee the efficacy and safety of biologics or biosimilars, it is inevitably to characterize the product-related impurities arising during the manufacturing process.
As regulatory agencies like FDA or EMA expect charge variants to be thoroughly characterized, a detailed analysis of deamidation, amidation, sialylation or pyroglutamate and lysin variants is of upmost importance during the development and commercialization of biologics.
The insights gained from these studies not only provide necessary knowledge about the product quality and safety, but also guides the development of process control strategies to avoid or reduce undesired charge variants.
In accordance with your needs, we adapt and, if required, qualify (according to ICH guidelines) our platform methods tailored to your drug substance (DS), its individual formulation matrix and your distinct analytical question.
Get in contact with us to quickly find the optimal setup for your analytical challenge and to speak directly from expert to expert.
Our capabilities
- How does charge heterogeneity analysis by cIEF or CZE distinguish C-terminal lysine containing antibody variants?
- How can charge isoforms detected by CE be identified by mass spectrometry?
- Which ICH parameters (i.e. Precision, Range, Limit of Quantification) do I need to qualify or validate when submitting my data to the regulatory authorities?
- Should I analyze my DS with IEX or cIEF for charge heterogeneity determination?
- Sourcing of reference products (Originators)
- Comparability studies
- Stability studies
- Accelerated stability studies
- Forced degradation studies
Standalone and integral bioanalytical services
- Amidation
- Deamidation
- Glycation
- Isomerization
- lysine clipping at C-terminus
- Oxidation
- Pyroglutamate
- Protein fragments
- Ion exchange chromatography (IEX-UV)
- Cation exchange chromatography coupled to mass spectrometry (CEX-MS)
- Isoelectric focusing (IEF)
- Capillary isoelectric focusing (cIEF) incl. determination of pI
- Capillary zone electrophoresis (CZE)
Tell us about your project
We‘ll advice you to define your assay needs
Technologies used
- LC-ESI-TOF
- Ion exchange chromatography (IEX-UV)
- Cation exchange chromatography coupled to mass spectrometry (CEX-MS)
- Isoelectric focusing (IEF)
- Capillary isoelectric focusing (cIEF) incl. determination of pI
- Capillary zone electrophoresis (CZE)
Please reach out to Our expert Dr. Anja Doebbe for all inquiries related to the characterization of charge heterogeneities.
Phone: +49 (0)521 329 363 47
Mail: Anja.Doebbe@Biofidus.com
