FDA – Waives Clinical Efficacy Studies
A New Era for Biosimilars: Powered by Analytics In a historic regulatory shift, the U.S. FDA has reportedly accepted the first application for a biosimilar monoclonal antibody without requiring a comparative clinical efficacy study. This landmark decision signals the beginning of a new era in biosimilar development, where scientific rigor and affordability converge. It also […]
A holistic analysis platform for assessing protein modification and functional activity

Linking Protein Modifications to Functional Impact. Protein modifications are critical for the efficacy, safety, and stability of therapeutic antibodies. Here, we introduce a comprehensive analysis platform tailored to assess post-translational modifications of therapeutic proteins hand in hand with their functional activity. In the present approach, we successfully applied peptide mapping with LC-ESI-MS to determine deamidation […]
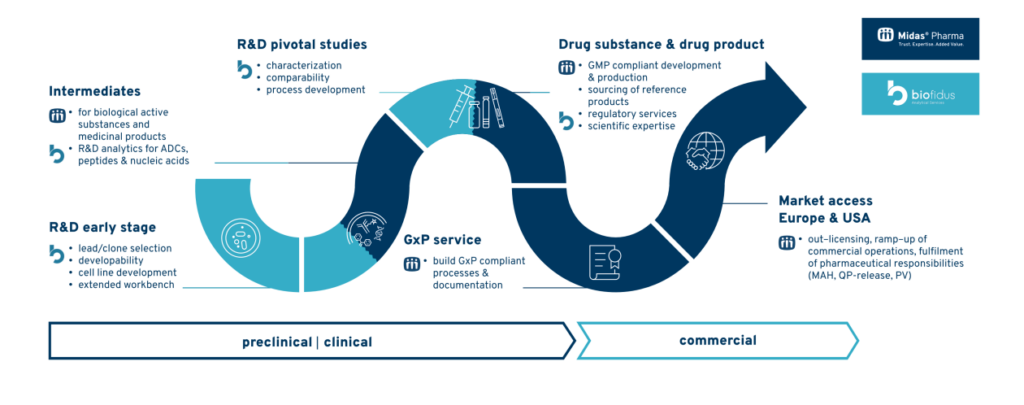
Midas Pharma and Biofidus join forces for integrated Biotech Solutions

Biofidus AG is now a member of the Midas group. With the acquisition of Biofidus, a leading provider of structural and functional characterization of biopharmaceuticals, Midas Pharma has taken a major step forward in expanding its portfolio of biopharmaceutical services. With this merger, the companies expand their portfolios of service solutions, offering seamless and modular […]