FDA – Waives Clinical Efficacy Studies
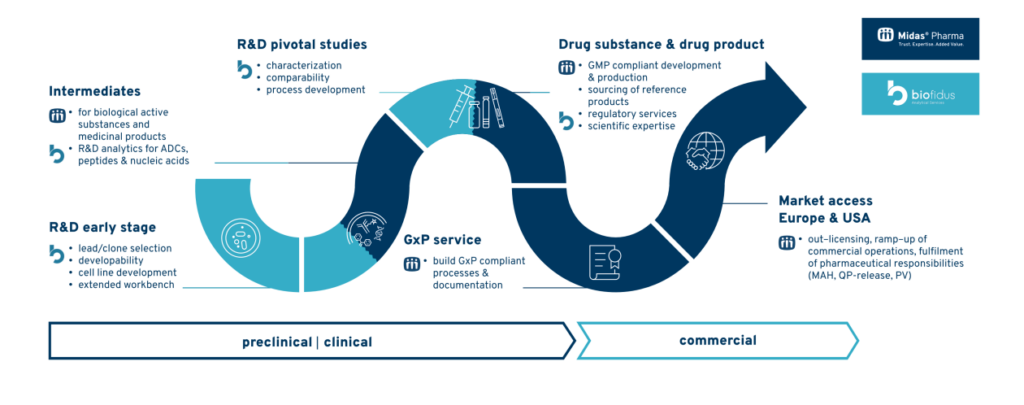
A New Era for Biosimilars: Powered by Analytics In a historic regulatory shift, the U.S. FDA has reportedly accepted the first application for a biosimilar monoclonal antibody without requiring a comparative clinical efficacy study. This landmark decision signals the beginning of a new era in biosimilar development, where scientific rigor and affordability converge. It also […]
